Abstract
Background: The canine is a highly appealing model for cancer research and discovery in part due to comparable histopathological features with humans, a fully intact immune system, similar clinicopathologic features, a more comparable body size and pharmacokinetic properties than the mouse, varied breed-specific incidence rates as well as a shared environment with humans. We and others have shown prominent transcriptomic overlap of human and canine NHL (cNHL) (McDonald T et al. Onctogarget, 2018). PI3K/Akt signaling plays an important role in lymphomagenesis, which is also a promising therapeutic target. However, identification of predictive genetic aberrations of therapeutic efficacy remains elusive. We evaluated the clinical activity of the pan-PI3K inhibitor, buparlisib, in a pilot clinical study in cNHL.
Methods:We enrolled and treated 10 dogs with buparlisibwho were diagnosed with BCL in an IRB and IACUC approved clinical study. Cases included 2 treatment naïve and 8 dogs with relapsed disease that had relapsed s/p CHOP (6), L' asparaginase (1) and VELCAP (1) treatment. Pet owners were consented and the study subjects received buparlisib9mg/kg orally for 28 consecutive days. Analysis for tumor response were evaluated on weekly basis through direct tumor measurement or use of x-rays. Post-therapy fine needle aspirates (FNA) were collected on Days 0, 7 and 21 to examine predictors of response to BKM120. RNA from fine need aspirate cells were isolated and the transcriptomic changes were evaluated using Canine Genome 2.0 Affymetrix Array, followed by unbiased systems biology assessment for biological pathways using Gene Set Enrichment Analysis (GSEA) and Ingenuity Pathway Analysis (IPA). We performed unbiased assessment to determine pertinent biological pathways associated with treatment response. The overall impact was used to determine the global effect on tumor progression and cancer risk based on the specific regulation of each gene. A Carcinogenic Risk Score (CRS) was calculated based on these values to determine if there is a promoted risk for cancer (positive value) or inhibitory risk for cancer (negative value) by summing the log2 fold-change values of key genes and subtracting this from the sum of log2 fold-change values of the tumor suppressors when comparing pre-treated to BKM120 treated dogs.
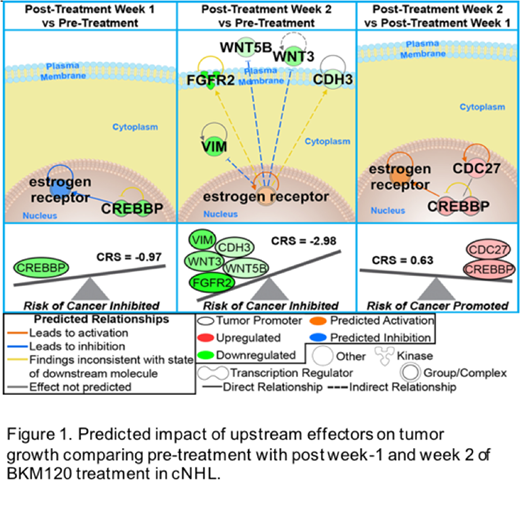
Results: Following four weeks of BKM120 treatment, the overall response rate was 30% with 1 complete response lasting 42 days; 2 partial responses lasting 55 and 72 days; 3 stable disease; and 4 progressive disease. Mild treatment related toxicities such elevated blood glucose, thrombocytopenia and anemia, fever, nausea and lethargic symptoms, with no treatment related toxicities in 2 cases were noted. Principal Component Analysis (PCA) and hierrachical clustering analysis of differentially expressed genes show that differentially expressed genes to cluster together in all dogs during post 2 week, indicating a consistent biological activity by BKM120 in all dogs regardless of breed, prior treatment or disease status. Pathway network analysis based on differentially expressed genes predicted activation of upstream regulators associated with tumor suppression including SOX1, SOX3 and GMNN (Week 1) and CEBPA (Week 2). Analysis of "key genes" involved in multiple biological processes appeared to be associated with response of PI3K inhibitortreatment. This included down regulation of CREBBP with a Cancer Risk Score (CRS) of -0.97 and downregulation of VIM, CDH3, WNT3, WNT5B and FGFR2 with a CRS of -2.98 (Fig 1).
Conclusion: Results from our pilot study in cNHL showed encouraging clinical responses with a pan-PI3K inhibitor in 3 of 10 dogs. Furthermore, our unbiased characterization of biological pathways revealed that the observed GEP changes associated with tumor suppression and they reduced the risk for cancer progression. Overall, the canine model appears to be particularly attractive model that may be leveraged for the study of clinical and biological responses to novel therapeutic oncologic agents.
Evens:Bayer: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Novartis: Consultancy; Acerta: Consultancy; Seattle Genetics, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics International DMC: Membership on an entity's Board of Directors or advisory committees; Tesaro: Research Funding; Janssen: Consultancy; Affimed: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal